In a subset analysis of the blinded phase of HPTN 083
Bone mineral density changes with APRETUDE vs TRUVADA
(SECONDARY ENDPOINT)
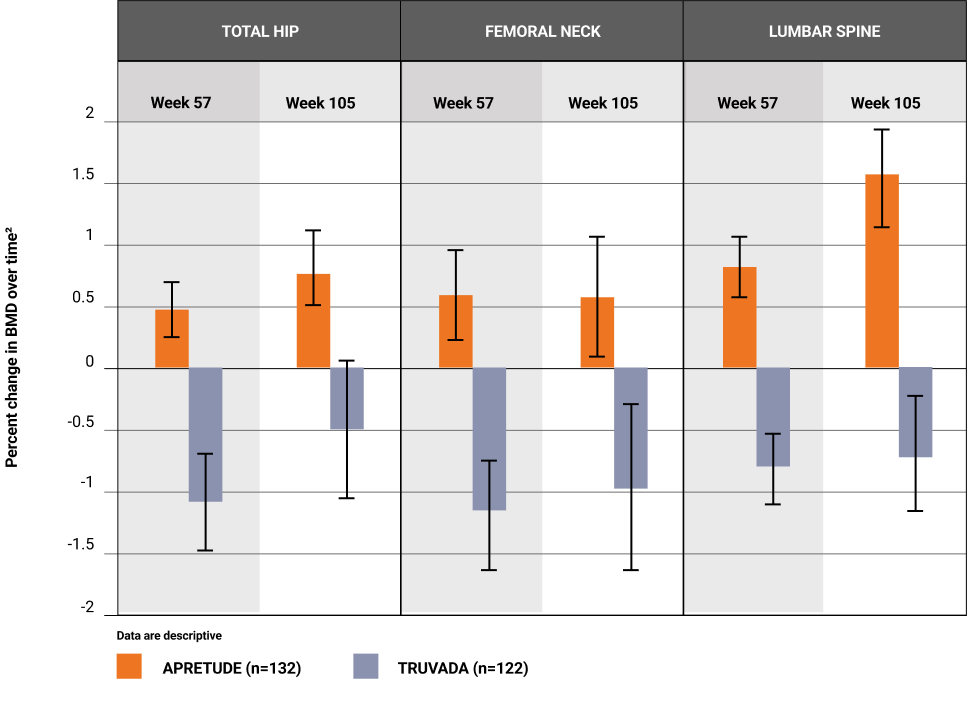
- A statistically significant difference* in bone mineral density that favored APRETUDE was seen between the 2 treatment arms at Weeks 57 and 1052
- Overall, no bone mineral density loss was observed in the APRETUDE treatment group at Weeks 57 and 1052
- A loss in bone mineral density by DXA scans was observed in the TRUVADA arm, consistent with previous findings with other TDF-containing regimens2,3
- The long-term clinical significance of these changes is not known (eg, fracture risk).2
*Adjusted for age and race.
BMD=bone mineral density; DXA=dual-energy X-ray absorptiometry; TDF=tenofovir disoproxil fumarate.
Number at risk (HPTN 083)1
The decreasing number at risk reflects the staggered enrollment of the study, the removal of participants when they experienced the event/outcome of interest, and when the blinded phase of the trial ended early.
Number at risk (HPTN 084)5
The decreasing number at risk reflects the staggered enrollment of the study, the removal of participants when they experienced the event/outcome of interest, and when the blinded phase of the trial ended early.
HPTN 083 Safety
Cisgender men and transgender women
SAFETY PROFILE ESTABLISHED IN >4500 PARTICIPANTS
Adverse drug reactions*: All grades reported in ≥1% in APRETUDE arm
6% of participants receiving APRETUDE and 4% of participants receiving TRUVADA discontinued due to adverse events (all causality).
*Adverse reactions defined as “treatment-related” as assessed by the investigator, with the exception of ISRs, where all ISRs were reported regardless of causality.
†Participants who received injection: APRETUDE (n=2117) and TRUVADA (n=2081).
‡Pyrexia includes pyrexia, feeling hot, chills, and influenza-like illness.
§Fatigue includes fatigue and malaise.
¶Sleep disorders include insomnia and abnormal dreams.
#Abdominal pain includes abdominal pain and upper abdominal pain.
HPTN=HIV Prevention Trials Network; ISR=injection-site reaction.
ISR breakdown
Most ISRs were mild to moderate and frequency and severity of reported ISRs decreased over time†
- The majority (97%) were Grade 1 or 2; 3% were Grade 3; none were Grade 4
- The median duration was 4 days
- 3% of participants discontinued APRETUDE due to ISRs
*Participants in the TRUVADA arm received placebo injections (matching vehicle, identical volume) during the double-blind phase of the study.1
†Self-reported ISRs could potentially underestimate the true rate of ISRs over time. ISRs may still be present but not reported during the course of the study.
Grade 1=mild; Grade 2=moderate; Grade 3=severe; Grade 4=a potentially life-threatening event.
ISR=injection-site reaction.
Frequency of reported ISRs decreased over time
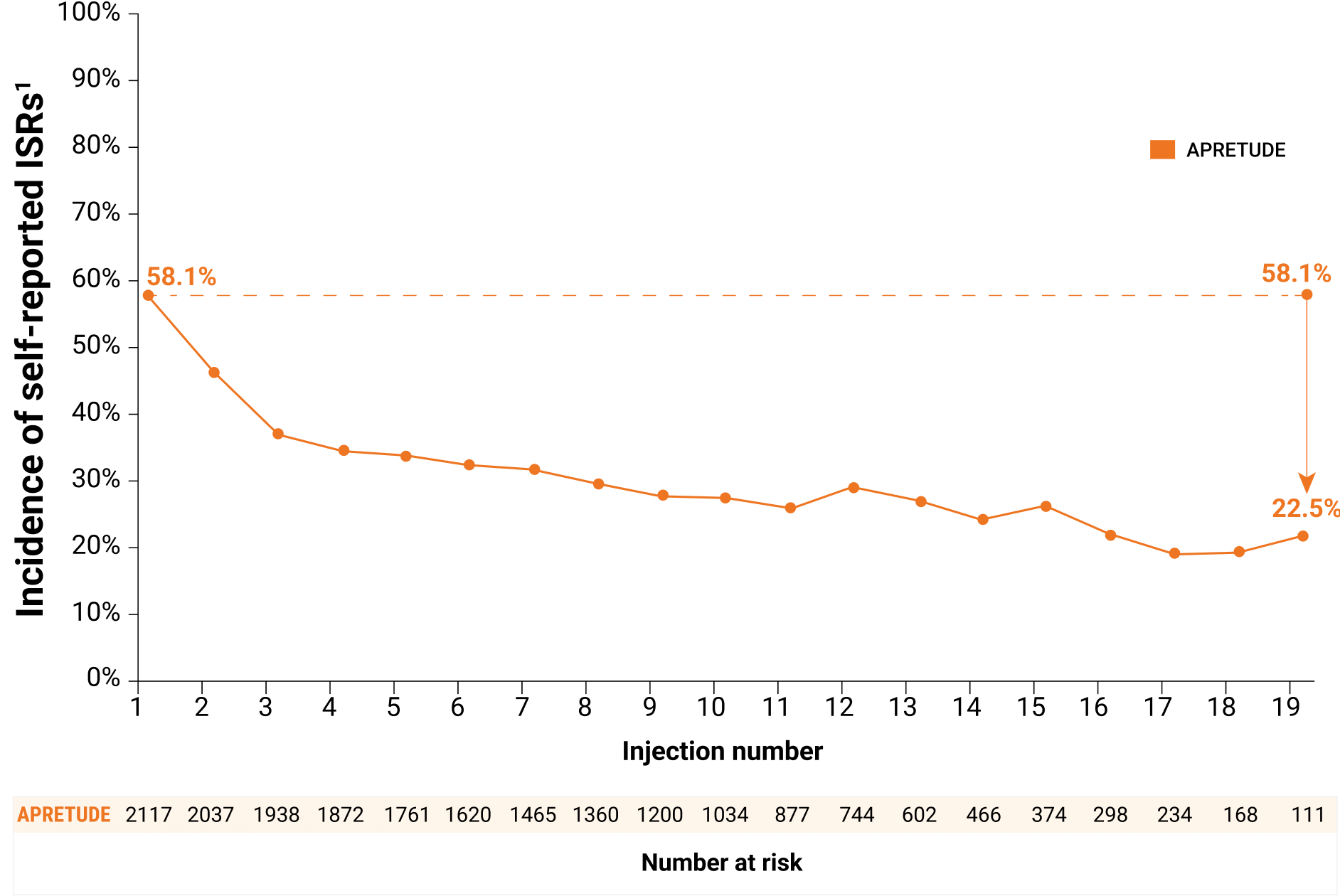
Self-reported ISRs could potentially underestimate the true rate of ISRs over time. ISRs may still be present but not reported during the course of the study.
HPTN 084 Safety
Cisgender women
SAFETY PROFILE ESTABLISHED IN >3200 PARTICIPANTS
Adverse drug reactions*: All grades reported in ≥1% in APRETUDE arm
1% of participants receiving APRETUDE and 1% of participants receiving TRUVADA discontinued due to adverse events (all causality).
*Adverse reactions defined as “treatment-related” as assessed by the investigator, with the exception of ISRs, where all ISRs were reported regardless of causality.
†Participants who received injection: APRETUDE (n=1519) and TRUVADA (n=1516).
‡Fatigue includes fatigue and malaise.
§Abdominal pain includes abdominal pain and upper abdominal pain.
¶Rash includes rash, erythema, pruritus, macular, papular, and maculopapular.
#Sleep disorders include insomnia and abnormal dreams.
ISR=injection-site reaction.
ISR breakdown
- Most ISRs were mild to moderate and frequency and severity of reported ISRs decreased over time†
- The majority (>99%) were Grade 1 or 2; <1% were Grade 3; none were Grade 4
- The median duration was 8 days
- No participants receiving APRETUDE discontinued due to ISRs
*Participants in the TRUVADA arm received placebo injections (matching vehicle, identical volume) during the double-blind phase of the study.4
†Self-reported ISRs could potentially underestimate the true rate of ISRs over time. ISRs may still be present but not reported during the course of the study.
Grade 1=mild; Grade 2=moderate; Grade 3=severe; Grade 4=a potentially life-threatening event.
ISR=injection-site reaction.
Frequency of reported ISRs decreased over time
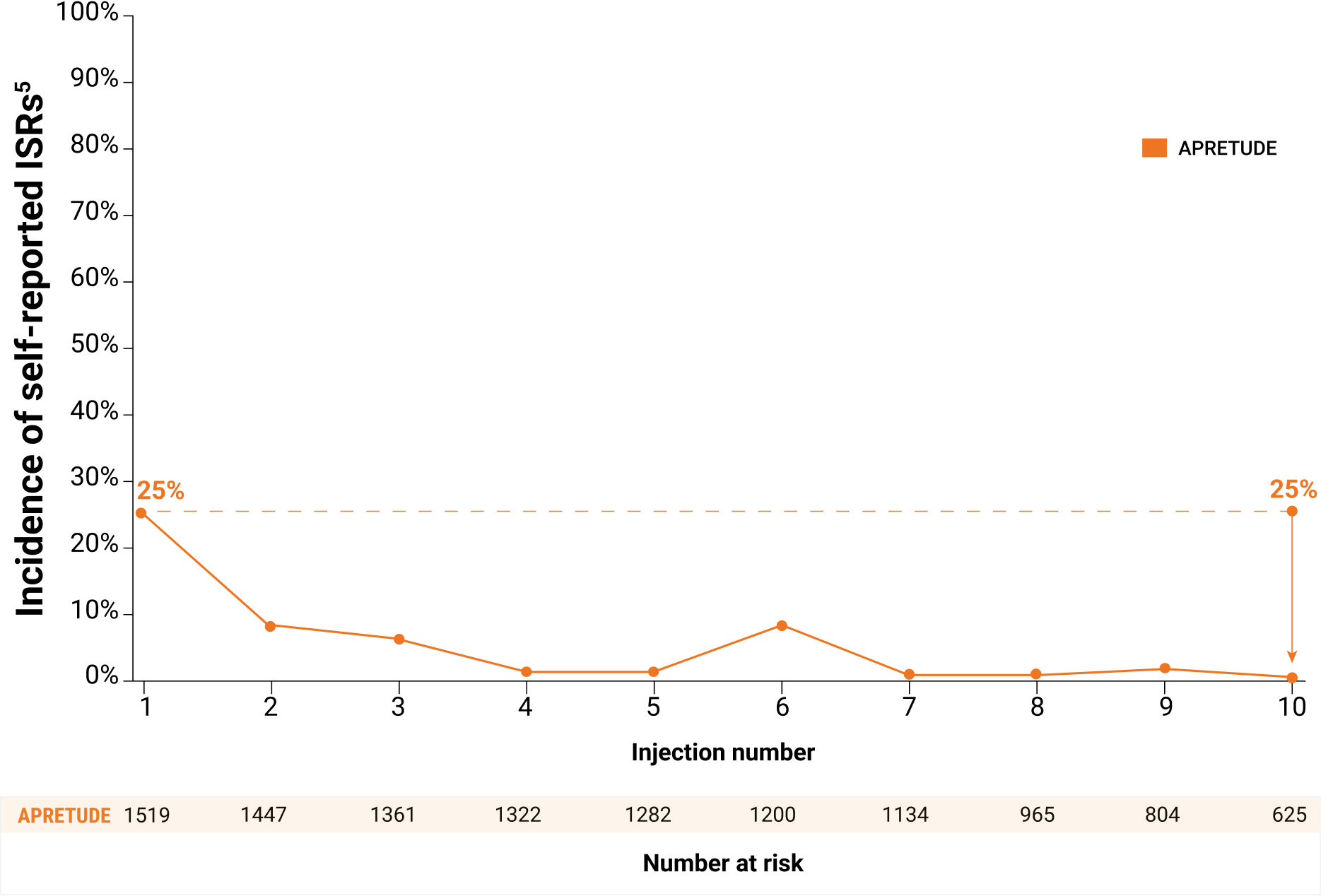
Self-reported ISRs could potentially underestimate the true rate of ISRs over time. ISRs may still be present but not reported during the course of the study.
PMUS-CBTWCNT250021
References:
Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595-608. doi:10.1056/NEJMoa2101016
Brown TT, Arao RF, Phanuphak N, et al. Bone density changes with CAB-LA or TDF/FTC PrEP in MSM and TGW in HPTN 083. Poster presented at: 30th Conference on Retroviruses and Opportunistic Infections; February 19-22, 2023; Seattle, WA. Accessed January 29, 2024. https://www.croiconference.org/abstract/bone-density-changes-with-cab-la-or-tdf-ftc-prep-in-msm-and-tgw-in-hptn-083
Baranek B, Wang S, Cheung AM, Mishra S, Tan DHS. The effect of tenofovir disoproxil fumarate on bone mineral density: a systemic review and meta-analysis. Antivir Ther. 2020;25(1):21-32. doi:10.3851/IMP334633-1
Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022;399(10337):1779-1789. doi:10.1016/ S0140-6736(22)00538-4
Data on file, ViiV Healthcare.



